Clinical Trials at Kugler Vision
Kugler Vision in Omaha, Nebraska, is a leader in ophthalmology and refractive surgery and is pleased to bring life-changing laser vision correction technology to the area. Our mission is to remain on the forefront of scientific advancements that improve our patients’ lives, and we are excited to offer new vision technology. Read more on the clinical trials Kugler Vision has had the privilege to conduct.
____________________________________________________________________________________________________________________________________________________________________________________________________________________________________
Past Clinical Trial: EVO ICL Trial
EVO Implantable Lens® Trial
Clinical Study for Correction of Nearsightedness and Astigmatism
We are excited to be clinical investigators for the EVO Implantable Lens® clinical study. The EVO procedure is designed to improve sight by treating a wide range of eyeglass or contact lens prescriptions.
Made from Collamer®, a unique implantable material, the replaceable lens works naturally with your eye to improve vision – without changing your cornea.
How EVO Works
If you suffer from nearsightedness or astigmatism, the EVO biocompatible lens is designed to improve your distance vision without glasses or contact lenses. Your eyeglass prescription can usually tell you how much nearsightedness and/or astigmatism you have
The EVO biocompatible lens is placed behind your iris (the colored part of your eye) and in front of the natural lens. The EVO lens focuses light on the back surface of your eye to improve your vision. The EVO procedure does not involve removing tissue from the cornea. The EVO procedure is additive, and works in harmony with your natural eye.
How to Participate in the EVO Implantable Lens® Clinical Study:
Consult with your eye doctor, who will conduct a thorough examination to find out whether you’re a candidate for the investigational EVO Implatable Lens and the EVO procedure.
If you would like to become an EVO Implantable Lens study participant, the first step is to contact us to set up an eye examination. Please call 402.558.2211 or email our team at [email protected].
Past Clinical Trial: IC-8 Clinical Lens Trial
Clinical Study for Cataract Patients
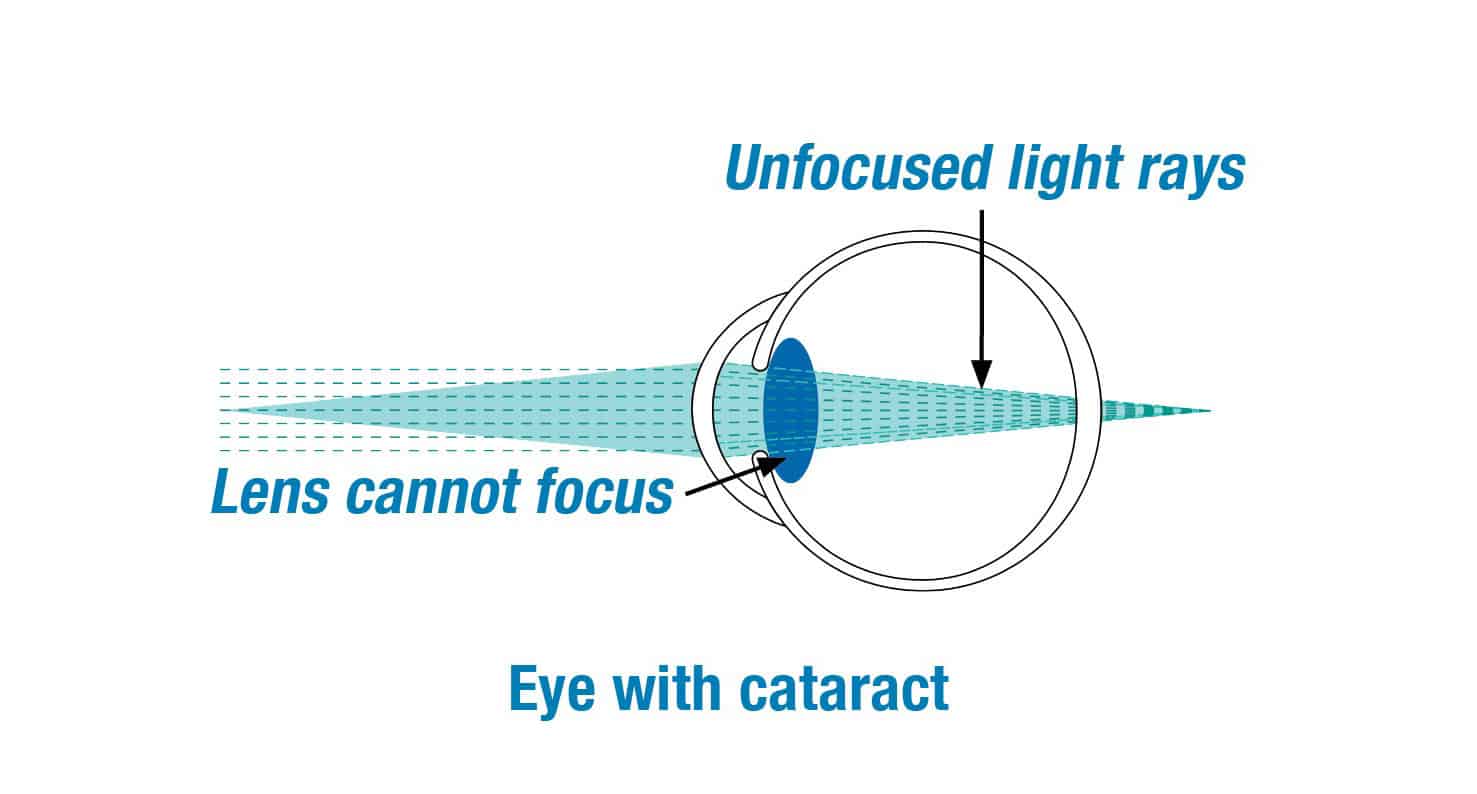
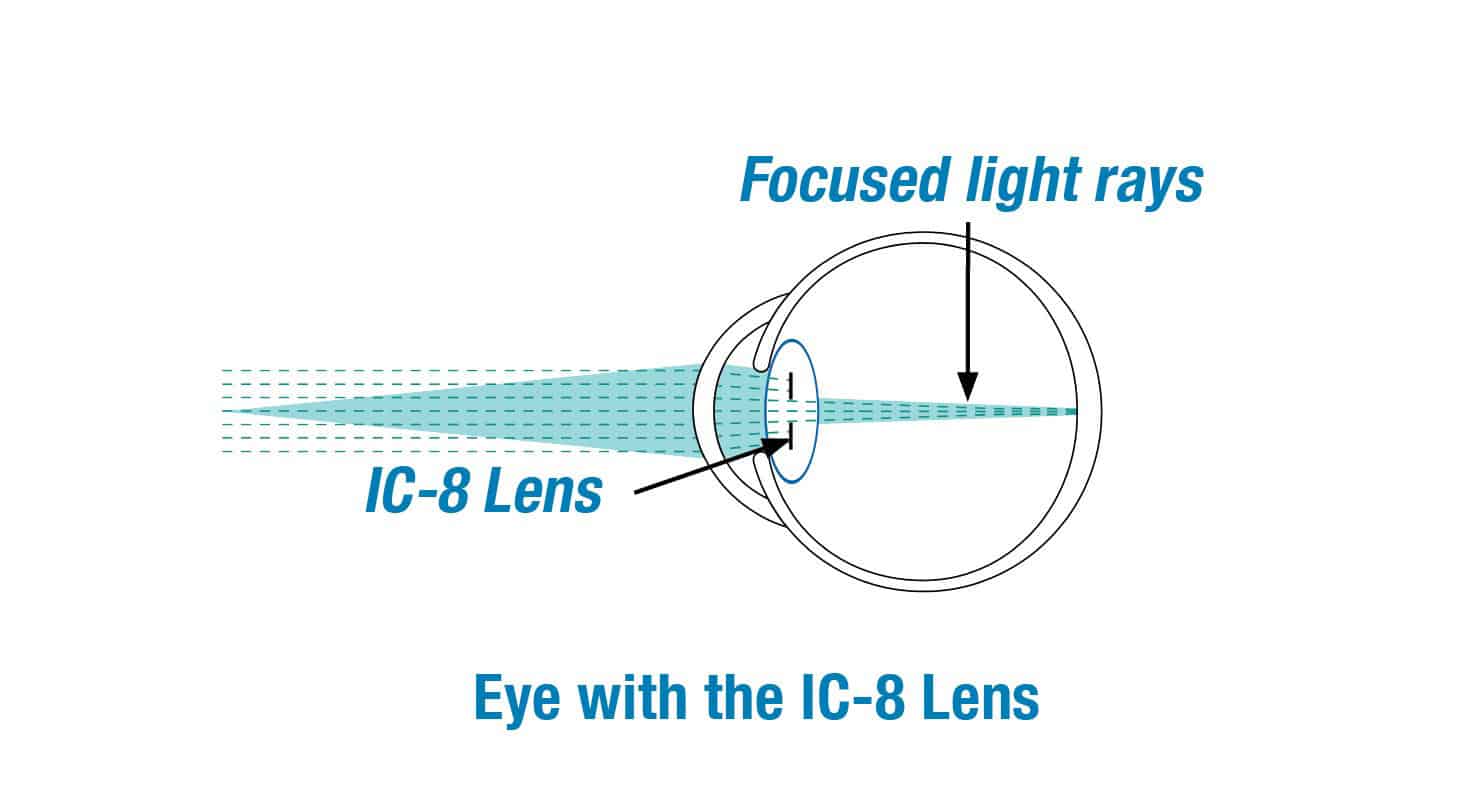
We are excited to be clinical investigators for the IC-8 lens clinical study. The clinical study is designed to evaluate if the IC-8 lens will provide far, intermediate and near vision for patients with cataracts.
The IC-8 lens is a clear monofocal lens with a mini-ring placed in the center. The mini-ring has an opening, or pinhole, designed to increase the range of vision by extending the focus of light rays that enter the eye.
 There are two ways you may be able to participate in the clinical study:
There are two ways you may be able to participate in the clinical study:
- Test Group (IC-8 Lens Group) will have a standard monofocal lens implanted in the first eye. If visual requirements are met after the first lens is implanted, the IC-8 lens will be implanted into the other eye. The IC-8 lens is an investigational device and is being studied to determine if vision at all distances is clearer than with a standard monofocal lens in both eyes.
- Control Group (Monofocal Lens Group) will have standard monofocal lens implanted in the first eye. If visual requirements are met, a standard monofocal lens will be implanted in the other eye. A standard monofocal lens is designed to provide far vision, but not near or intermediate vision.
 Caution: Investigational Device. Limited by Federal (or United States) law to investigational use.
Caution: Investigational Device. Limited by Federal (or United States) law to investigational use.
Past Clinical Trial: Corneal Crosslinking
On Nov. 7, 2012 , Kugler Vision became the first institution in Nebraska to perform the corneal cross-linking procedure to treat keratoconus. The procedure was done as part of national, multicenter clinical trials examining the safety and efficacy of the corneal cross-linking procedure for the treatment of keratoconus and corneal ectasia after refractive surgery.
What is keratoconus?
So, just what is keratoconus? Keratoconus is a progressive degenerative eye disease affecting one in every 200 to 500 Americans. Patients with this disease experience a gradual steepening and thinning of the cornea, which leads to a progressive loss of vision. Until now, the only treatment options were specialized hard contacts or INTACS implantation, until corneal transplant surgery was required. Although corneal transplantation is generally effective, it is a major surgery that carries risks and complications, including vision loss. Additionally, successful surgery still requires a recovery period of nine to 12 months.
What is corneal cross-linking?
Cross-linking attempts to reduce the progression of keratoconus or corneal ectasia by strengthening the collagen bonds inside the cornea. People who undergo the cross-linking procedure as part of the research study receive drops of riboflavin (vitamin B2) ophthalmic solution, a micronutrient that promotes collagen strengthening, that are activated through the use of ultraviolet light therapy.
The result is a stronger corneal structure that gives the cornea greater ability to withstand the degeneration that occurs in diseases such as keratoconus.
Lance Kugler, MD, was one of a small group of 100 principal investigators conducting a clinical trial evaluating the safety and efficacy of collagen corneal cross-linking in the United States.
This trial has concluded and Kugler Vision is no longer seeking subjects to participate in this national corneal cross-linking clinical trial to study the safety and efficacy of this treatment.
Past Clinical Trial: Presbyopia Correction Clinical Research Study
These clinical trials have concluded. Lance Kugler, MD, and Patti Fries, OD, coordinated with Refocus Group (www.refocus-group.com) to conduct clinical research studies to determine if the investigational Scleral Spacing Procedure (SSP) and the PresVIEW Scleral Implant (PSI) would eliminate the need for corrective eyewear arising from presbyopia.
What is presbyopia?
Presbyopia (which means “aging eye”) is an age-related eye condition that makes it more difficult to see up close, such as when you are reading. Like gray hair and wrinkles, presbyopia is a symptom caused by the natural course of aging, not a disease. The first signs of presbyopia are usually noticed between the ages of 40 and 50, and can include:
- difficulty focusing on fine print and small objects (particularly in low light)
- the need to hold reading materials farther away in order to focus
- eyestrain when reading for long periods
- momentary blurred vision when transitioning between viewing distances
Presbyopia differs from myopia (nearsightedness), hyperopia (farsightedness), and astigmatism, which are related to the shape of the eyeball and are caused by genetic and environmental factors. Unlike these commonly known eye disorders, presbyopia is simply a symptom of aging and eventually affects everyone.